A opioid withdrawal inhibitor’s trial questionable
As the crippling effects of the opioid epidemic continue to grip our nation’s people, we continue to search for effective means to combat the issue before it begin. Unfortunately, in wake of the current trends we must look for remedies before solutions.
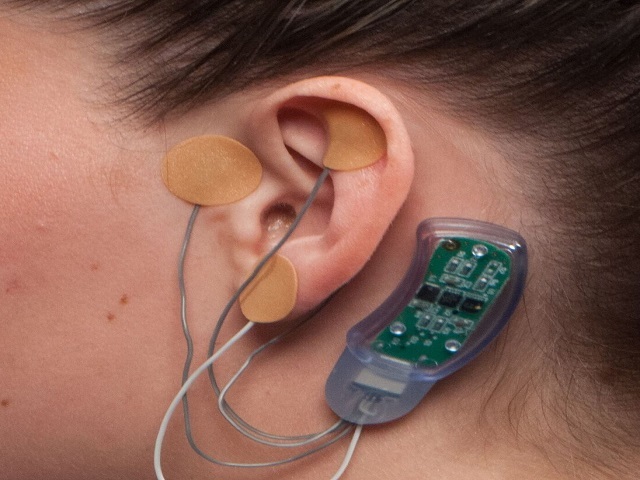
So in 2017, when Innovative Health Solutions proposed their “Bridge” device to the FDA claiming to be the “first non-pharmaceutical, non-implantable medical device available today for opiate (heroin) and opioid (e.g. oxycontin, methadone, suboxone) addicts needing help with withdrawal pain and associated symptoms,” it was given the green light.
However, it came to light Wednesday in an article by NPR and Side Effects Public Media, that researchers working with Innovative Health Solutions, submitted to a medical journal, a study that wasn’t what it appeared to be.
The device, which is placed behind the ear and sends small electrical pulses through four cranial nerves to treat withdrawal symptoms and chronic pain, was reportedly tested through a “retrospective study,” which means they would have simply reviewed existing medical data.
It was brought to light, however, that Innovative Health Solutions conducted a clinical trial that skirted FDA rules and ethical norms and ended up using the vulnerable people it was intended to help as test as involuntary test subjects.
The information from this alleged study was then later what the FDA relied upon when it made the decision to clear the Bridge for marketing as a treatment for opioid withdrawal.
When asked by Side Effects and NPR, the FDA said it “cleared the Bridge for use in opioid withdrawal because the study demonstrated a clinical benefit that outweighs the risk of the device.”
The agency also said it is reviewing the issues raised by the investigation but didn’t say what, if any, action it might take.

I am in 12th grade. I would like to become a Wildlife and Fisheries biologist. I love discussing the news. Learning people's opinions helps me form my...